First in Preimplantation Genetic Diagnosis in the United States
Led by Dr. Kangpu Xu, Cornell’s Preimplantation Genetics Laboratory has assisted in hundreds of CRM patients becoming parents to healthy babies following PGT (previously called PGD/PGS)—starting in 1993 with the conception of the first, healthy PGD baby in the United States.
The purpose of preimplantation genetic testing is to identify specific eggs and/or embryos that are affected with certain genetic disorders. Preimplantation genetic testing may be used in conjunction with in vitro fertilization (IVF) and serves for three clinical indications: preimplantation genetic testing for aneuploidy (PGT-A), for monogenic (single gene) disorders (PGT-M), and for chromosomal structural rearrangements (PGT-SR, most commonly seen as balanced translocations).
In order to perform preimplantation genetic testing, the embryo must be biopsied. Embryo biopsy is an invasive but safe procedure and is typically performed on day 5 or 6 of embryo development by removing a few cells from the trophectoderm with a microsurgical needle. The cells are then genetically analyzed for abnormalities. During the screening process, the embryo will be cryopreserved until the results are available.
The risks and benefits of preimplantation genetic testing vary among individuals. Testing procedures and techniques are specific to the patient and her needs. Your CRM physician will work with our genetic counseling team to design the optimal treatment plan.
Preimplantation Genetic Testing (PGT)
PGT may be used to screen eggs and/or embryos for a specific genetic disorder or for unbalanced chromosome products due to a parental chromosome abnormality. Indications for PGT include, but are not limited to, one or both intended parents being a carrier of a balanced chromosome translocation or inversion, having a known family history of genetic disorders, or being a carrier of or affected with a known genetic mutation and/or marker.
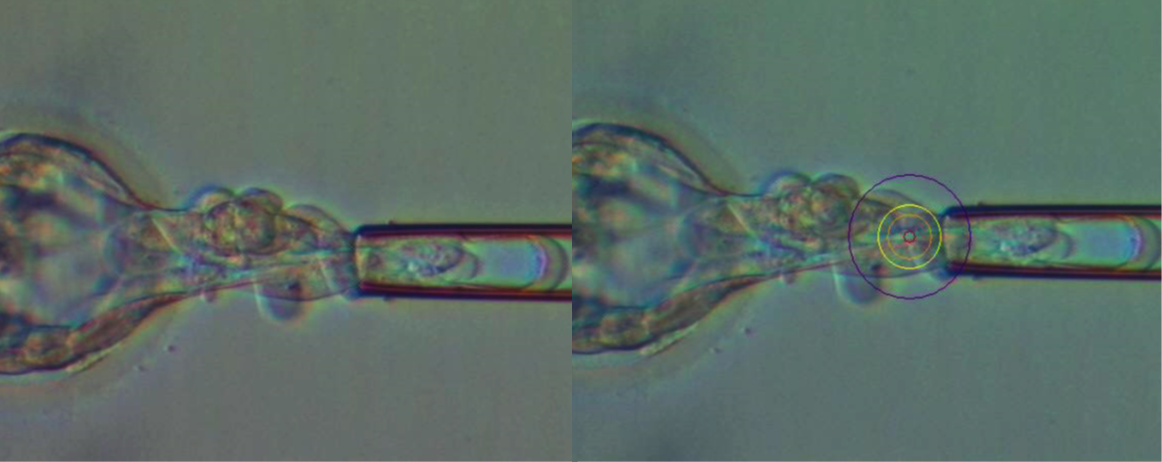
Above, Day 5 and 6 blastocyst biopsy with laser used to obtain a few cells.
PGT may benefit patients in the following situations:
- Sex-linked disease such as hemophilia
- Human leukocyte antigen (HLA) matching
- Family history of specific genetic disorders:
- Beta-thalassemia
- Cancer predisposition genes (BRCA1/2, Lynch Syndrome, and others)
- Cystic fibrosis
- Duchenne muscular dystrophy
- Fragile X syndrome
- Huntington disease
- Retinoblastoma
- Sickle cell anemia
- Tay-Sach disease
- And hundreds more
Preimplantation Genetic Testing for Aneuploidy (PGT-A)
It is clear that age related declining fertility is mainly due to decreasing of ovarian reserve and increasing of aneuploidy in their embryos (Figure Below). It is also well established that aneuploidy is a cause of implantation failure.
PGT-A may be used to screen eggs and/or embryos for chromosome abnormalities (Figure Below). Indications for PGT-A include advanced maternal age, multiple previous failed IVF attempts, repetitive miscarriage, or pregnancy with a chromosome abnormality. PGT-A may also be used to identify a single euploid embryo for transfer.
At CRM, Next Generation Sequencing (NGS) is exclusively used to screen for all 23 pairs of chromosomes. First, the whole genome is amplified from biopsied specimens. A library of each specimen with all pieces of amplified is prepared. The DNA sequences are determined in a NGS machine. Then, the data is imported to be evaluated by advanced analytic software against a known normal control. NGS has the capability to detect whole chromosome aneuploidy as well as large segments of duplications and deletions of chromosomes. (Figure Below) Furthermore, NGS may provide some information on mosaicism in the biopsied sample, allowing prioritization of embryos for transfer.
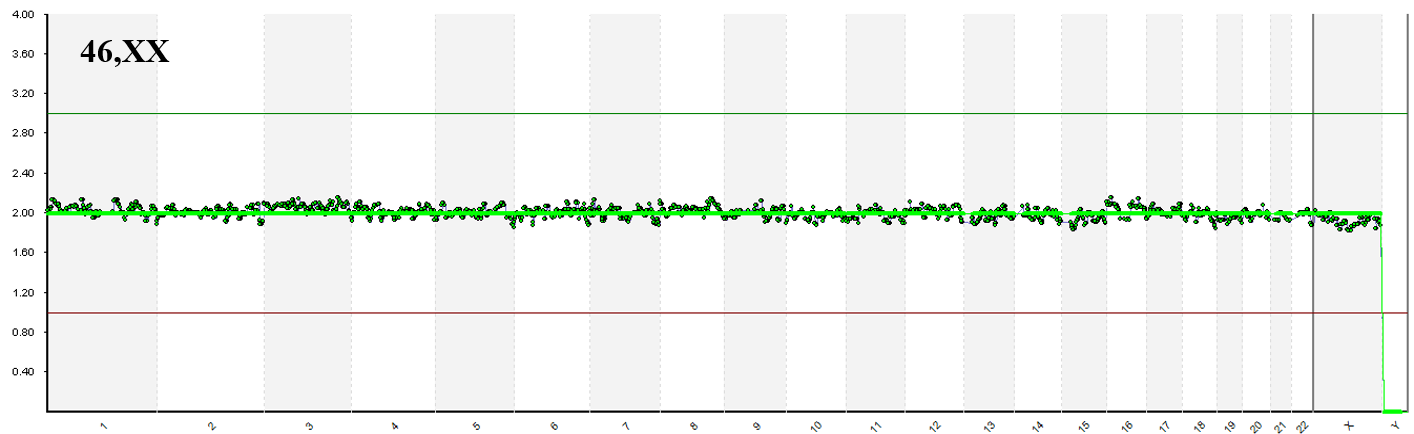
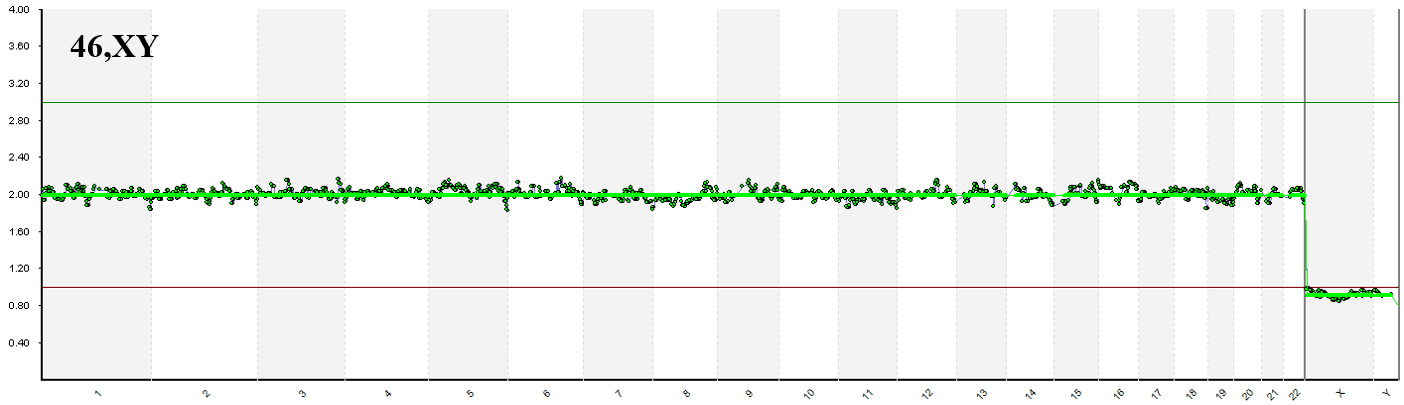
Normal 46,XX and 46,XY identified by Next Generation Sequencing
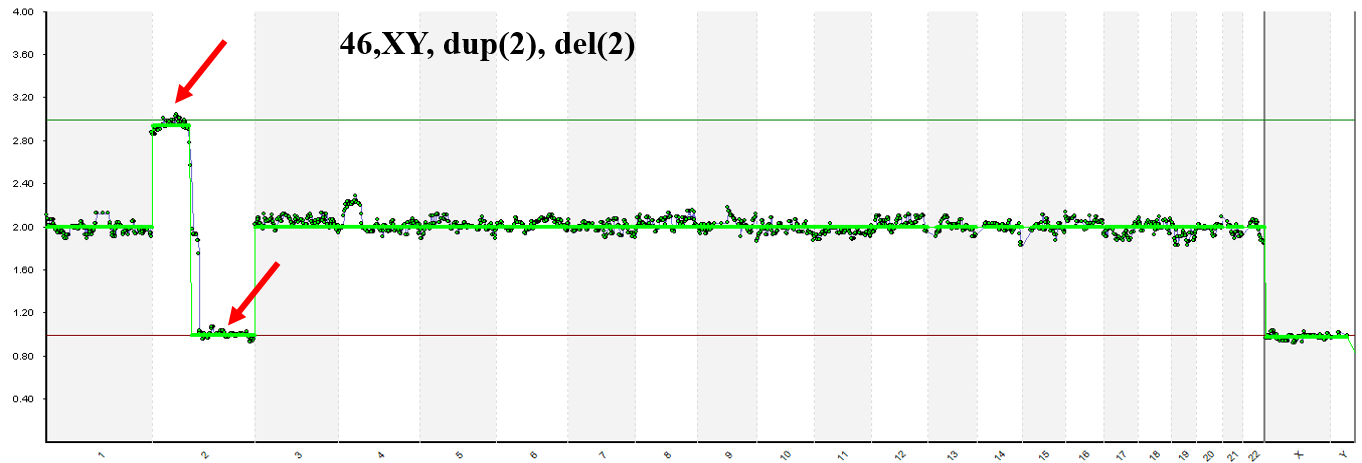
NGS detected segmental gain/loss on chromosome 2.

